Levodopa 50/12 5
Levodopa 50/12 5. LCE 50 Stalevo 50 125 mg 200 mg 50 mg Generic Name. MADOPAR capsules contain levodopa 200mg and benserazide 50mg. Madopar 625 capsules contain levodopa 50 mg and benserazide 125 mg.
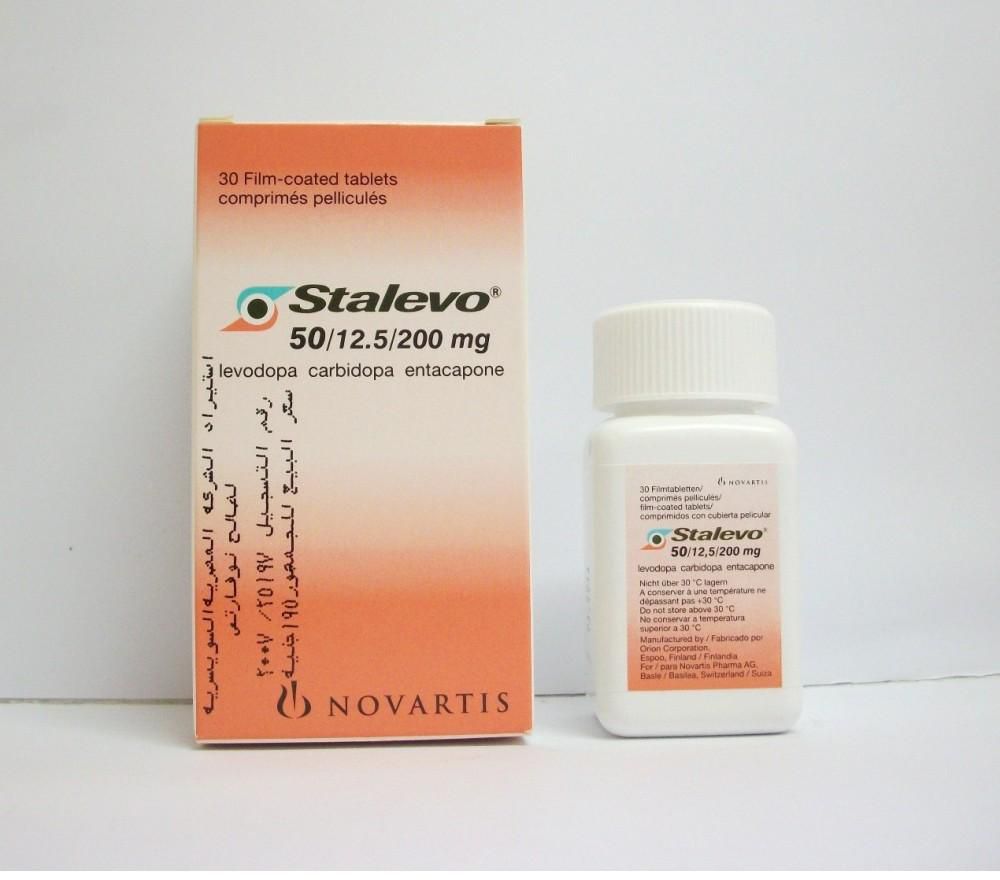
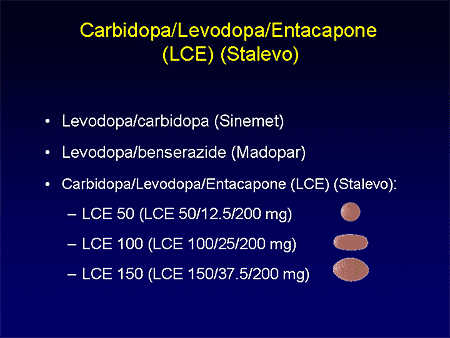
Stalevo 50125200 containing 125 mg carbidopa 50 mg levodopa and 200 mg entacapone. Controlled Release CR preparations contain the same amount of Levodopa as the immediate release preparations and the conversion to Madopar. Madopar 50125 dispersible tablets according to Table 2.
MADOPAR 625 capsules contain levodopa 50mg and benserazide 125mg. MADOPAR 125 capsules and MADOPAR HBS capsules contain levodopa 100mg and benserazide 25mg. Madopar HBS 125 slow-release capsules contain levodopa 100 mg and benserazide 25 mg.
Benserazide and Levodopa This information from Lexicomp explains what you need to know about this medication including what its used for how to take it its side effects and when to call your healthcare provider. Commonly used name generally brand name Generic drug names Dosage form basically what it says on the label. Stalevo is supplied in 4 strengths.
If the disease is at an advanced stage the starting dose should be one capsule or dispersible tablet of Madopar. Each tablet of Sinemet 10 mg100 mg Tablets contains 108 mg carbidopa equivalent to 10 mg of anhydrous carbidopa and 100 mg levodopa. There are 4 common formulations each of which has several available dosages.
Each tablet of Sinemet 125 mg50 mg Tablets contains 135 mg carbidopa equivalent to 125 mg of anhydrous carbidopa and 50 mg levodopa. Welche unerwünschten Wirkungen können auftreten. The dose of co-careldopa Sinemet is expressed with the carbidopa content first and then the levodopa content.
Carbidopa also helps to make sure you experience fewer side effects. Carbidopaentacaponelevodopa Pill with imprint LCE 50 is Brown Round and has been identified as Stalevo 50 125 mg 200 mg 50 mg.
Initially 50 mg 3 or 4 times daily 100 mg tid in advance stage disease.
Madopar 250 capsules contain levodopa 200 mg and benserazide 50 mg. Welche unerwünschten Wirkungen können auftreten. The recommended initial dose is one capsule or dispersible tablet of Madopar 50 mg125 mg three or four times daily. Stalevo 50125200 containing 125 mg carbidopa 50 mg levodopa and 200 mg entacapone. Nebenwirkungen von LEVODOPABenserazid-ratiopharm 50 mg125 mg Tabl. Madopar 50125 dispersible tablets according to Table 2. Controlled Release CR preparations contain the same amount of Levodopa as the immediate release preparations and the conversion to Madopar. The carbidopa ingredient helps levodopa get in to the brain where it can become dopamine. The dose of co-careldopa Sinemet is expressed with the carbidopa content first and then the levodopa content.
Benserazide and Levodopa This information from Lexicomp explains what you need to know about this medication including what its used for how to take it its side effects and when to call your healthcare provider. Madopar 250 capsules contain levodopa 200 mg and benserazide 50 mg. The recommended initial dose is one capsule or dispersible tablet of Madopar 50 mg125 mg three or four times daily. LCE 50 Stalevo 50 125 mg 200 mg 50 mg Generic Name. Madopar HBS 125 slow-release capsules contain levodopa 100 mg and benserazide 25 mg. There are 4 common formulations each of which has several available dosages. The dose of co-careldopa Sinemet is expressed with the carbidopa content first and then the levodopa content.


































Post a Comment for "Levodopa 50/12 5"